Metal complexes as “protein surface mimetics”
Abstract
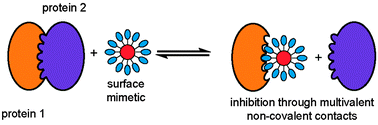
A key challenge in chemical biology is to identify small molecule regulators for every single protein. However, protein surfaces are notoriously difficult to recognise with synthetic molecules, often having large flat surfaces that are poorly matched to traditional small molecules. In the surface mimetic approach, a supramolecular scaffold is used to project recognition groups in such a manner as to make multivalent non-covalent contacts over a large area of protein surface. Metal based supramolecular scaffolds offer unique advantages over conventional organic molecules for protein binding, including greater stereochemical and geometrical diversity conferred through the metal centre and the potential for direct assessment of binding properties and even visualisation in cells without recourse to further functionalisation. This feature article will highlight the current state of the art in protein surface recognition using metal complexes as surface mimetics.

 Please wait while we load your content...
Please wait while we load your content...