Double C–H amination by consecutive SET oxidations†
Abstract
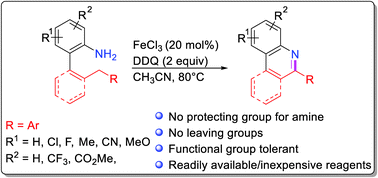
A new method for intramolecular C–H oxidative amination is based on a FeCl3-mediated oxidative reaction of anilines with activated sp3 C–H bonds. The amino group plays multiple roles in the reaction cascade: (1) as the activating group in single-electron-transfer (SET) oxidation process, (2) as a directing group in benzylic/allylic C–H activation at a remote position, and (3) internal nucleophile trapping reactive intermediates formed from the C–H activation steps. These multielectron oxidation reactions proceed with catalytic amounts of Fe(III) and inexpensive reagents.

 Please wait while we load your content...
Please wait while we load your content...