Nickel(0)-catalyzed intramolecular reductive coupling of alkenes and aldehydes or ketones with hydrosilanes†
Abstract
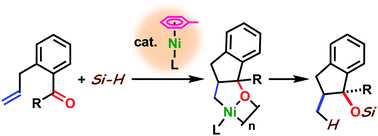
A nickel(0)-catalyzed reductive coupling of aldehydes and simple alkenes with hydrosilanes has been developed. A variety of silyl-protected 1-indanol derivatives were prepared in a highly diastereoselective manner (up to >99 : 1 dr) by employing a combination of nickel(0)/N-heterocyclic carbene and triethylsilane. The present system was also applied to a reductive coupling with ketones. Preliminary results of a nickel(0)-catalyzed asymmetric three-component coupling reaction of an aldehyde, an alkene, and triethylsilane are also shown.

 Please wait while we load your content...
Please wait while we load your content...