Copper-mediated C(sp3)–H azidation with Me3SiN3: synthesis of imidazoles from ketones and aldehydes†
Abstract
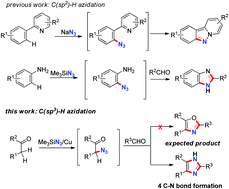
The efficient construction of 2,4,5-trisubstituted imidazoles, through a copper-mediated three-component reaction involving ketones, aldehydes, and Me3SiN3, has been developed. During the process, 4 C–N bonds were formed sequentially. Experimental results and DFT calculations suggested that azidation of the alpha methylene group of the ketone was the key C–N bond-forming step.

 Please wait while we load your content...
Please wait while we load your content...