Transition-metal-free cascade reaction of α-halo-N-tosylhydrazones, indoles and arylboronic acids†
Abstract
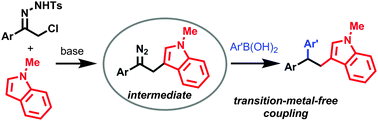
α-Halo-N-tosylhydrazones are employed as reagents for the formation of multiple carbon–carbon bonds in the three-component reactions. In this transformation, a strategy has been designed to generate the diazo intermediate by using a nucleophile to react with the azoalkene intermediate generated in situ from the α-halo-N-tosylhydrazone. The diazo intermediate thus generated further undergoes transition-metal-free C–C bond forming reaction with arylboronic acids.

 Please wait while we load your content...
Please wait while we load your content...