Intermolecular dearomative C2-arylation of N-Ac indoles activated by FeCl3†
Abstract
We report the FeCl3-mediated direct addition of electron-rich arenes to the C2-position of electrophilic N-Ac indoles under mild conditions (room temperature, air). No functional group is required on the arene nucleophile: one of its C–H bonds is added to the C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 double bond of the indole nucleus in a Friedel–Crafts-type reaction. This dearomatisation process delivered a broad range of C2-arylated indolines.
C3 double bond of the indole nucleus in a Friedel–Crafts-type reaction. This dearomatisation process delivered a broad range of C2-arylated indolines.
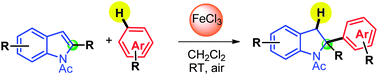


 Please wait while we load your content...
Please wait while we load your content...