A mechanistic insight into metal-cluster π-envelopment: a dual binding mode involving bent and planar ligand-conformers†
Abstract
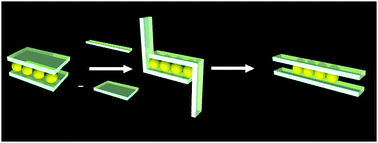
Metal clusters are effectively stabilized by bridging π-coordination of planar π-conjugated unsaturated hydrocarbons. However, the mechanism of π-envelopment of a metal cluster has been elusive. By employing 1,2-bis(4-aryl-1,3-butadienyl)benzene as the π-conjugated ligand, we found that the π-envelopment of a Pd4 cluster proceeded in a stepwise manner, where the sp2-carbon ligands initially envelop the Pd4 cluster through a bent binding mode, and then isomerized to a thermodynamically more stable planar mode under mild heating or visible light irradiation. The involvement of a bent binding mode indicates the kinetically preferred coordination at the axial coordination site trans to a metal–metal bond.

 Please wait while we load your content...
Please wait while we load your content...