Redox condensation of o-halonitrobenzene with 1,2,3,4-tetrahydroisoquinoline: involvement of an unexpected auto-catalyzed redox cascade†
Abstract
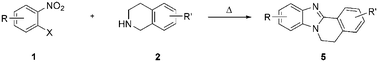
A practical synthesis of fused benzimidazoles 5 has been developed by simply heating o-halonitrobenzenes 1 with tetrahydroisoquinolines 2. In this transformation, 2 played multiple roles as a building block, base and a double hydride donor in a cascade of uncatalyzed aromatic substitution, reduction of the nitro group, oxidation of the α-methylene group and condensation.

 Please wait while we load your content...
Please wait while we load your content...