Structural studies of β-turn-containing peptide catalysts for atroposelective quinazolinone bromination†
Abstract
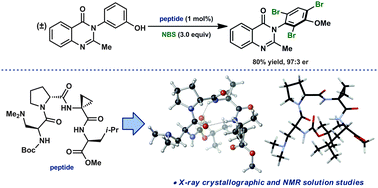
We describe herein a crystallographic and NMR study of the secondary structural attributes of a β-turn-containing tetra-peptide, Boc-Dmaa-D-Pro-Acpc-Leu-NMe2, which was recently reported as a highly effective catalyst in the atroposelective bromination of 3-arylquinazolin-4(3H)-ones. Inquiries pertaining to the functional consequences of residue substitutions led to the discovery of a more selective catalyst, Boc-Dmaa-D-Pro-Acpc-Leu-OMe, the structure of which was also explored. This new lead catalyst was found to exhibit a type I′ β-turn secondary structure both in the solid state and in solution, a structure that was shown to be an accessible conformation of the previously reported catalyst, as well.
- This article is part of the themed collection: Foldamers

 Please wait while we load your content...
Please wait while we load your content...