Stabilization of a Zn(ii) hydrosulfido complex utilizing a hydrogen-bond accepting ligand†
Abstract
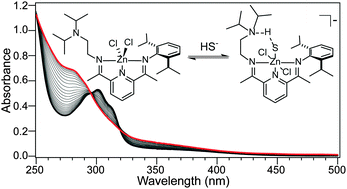
Hydrogen sulfide (H2S) has gained recent attention as an important biological analyte that interacts with bioinorganic targets. Despite this importance, stable H2S or HS− adducts of bioinorganic metal complexes remain rare due to the redox activity of sulfide and its propensity to form insoluble metal sulfides. We report here reversible coordination of HS− to Zn(didpa)Cl2, which is enabled by an intramolecular hydrogen bond between the zinc hydrosulfido product and the pendant tertiary amine of the didpa ligand.

 Please wait while we load your content...
Please wait while we load your content...