Deciphering the roles of multiple additives in organocatalyzed Michael additions†
Abstract
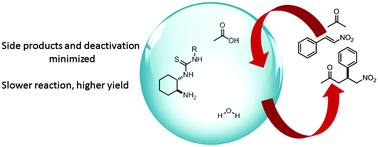
The synergistic effects of multiple additives (water and acetic acid) on the asymmetric Michael addition of acetone to nitrostyrene catalyzed by primary amine-thioureas (PAT) were precisely determined. Acetic acid facilitates hydrolysis of the imine intermediates, thus leading to catalytic behavior, and minimizes the formation of the double addition side product. In contrast, water slows down the reaction but minimizes catalyst deactivation, eventually leading to higher final yields.

 Please wait while we load your content...
Please wait while we load your content...