Ligand-controlled product selectivity in palladium-catalyzed domino post-Ugi construction of (spiro)polyheterocycles†
Abstract
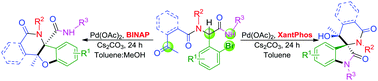
A novel diversity-oriented access to (spiro)polyheterocycles possessing two adjacent quaternary carbon stereocenters is described. By combining an Ugi 4-CR with Pd-catalysis, the possible mode of cyclization is controlled by the appropriate choice of ligands. Using XantPhos, spirooxindoles are selectively generated, while with BINAP fused polycyclic cis-dihydrobenzofurans are obtained with high diastereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...