Towards vast libraries of scaffold-diverse, conformationally constrained oligomers
Abstract
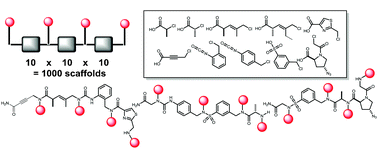
There is great interest in the development of probe molecules and drug leads that would bind tightly and selectively to protein surfaces that are difficult to target with traditional molecules, such as those involved in protein–protein interactions. The currently available evidence suggests that this will require molecules that are larger and have quite different chemical properties than typical Lipinski-compliant molecules that target enzyme active sites. We describe here efforts to develop vast libraries of conformationally constrained oligomers as a potentially rich source of these molecules.
- This article is part of the themed collection: Foldamers

 Please wait while we load your content...
Please wait while we load your content...