Why does Togni's reagent I exist in the high-energy hypervalent iodine form? Re-evaluation of benziodoxole based hypervalent iodine reagents†
Abstract
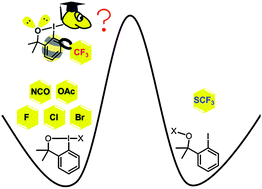
Togni's reagents have become very popular trifluoromethylating reagents in organic synthesis. The existing form of Togni's reagent I is a hypervalent iodine compound which lies much higher in energy than its ether isomer. The high-energy hypervalent iodine form makes Togni's reagent I very effective and versatile. The energy differences between the two forms correlate with the trans influence of the substituents. The five-membered ring in the benziodoxole-based scaffold is an important reason for its existence in the higher-energy form. The relation to Buchwald's 2014 research is discussed.

 Please wait while we load your content...
Please wait while we load your content...