Hydrogen bonding to carbonyl oxygen of nitrogen-pyramidalized amide – detection of pyramidalization direction preference by vibrational circular dichroism spectroscopy†
Abstract
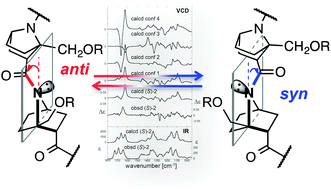
Nitrogen-pyramidalization of amide increases electron density on nitrogen and decreases that on carbonyl oxygen. We identified hydrogen-bonding to carbonyl of nitrogen-pyramidalized bicyclic β-proline derivatives by crystallography, and by NMR and vibrational circular dichroism (VCD) spectroscopy in solution. Such hydrogen-bonding can switch the preferred nitrogen-pyramidalization direction, as detected by VCD spectroscopy.
- This article is part of the themed collection: Foldamers

 Please wait while we load your content...
Please wait while we load your content...