The cubyl cation rearrangements†
Abstract
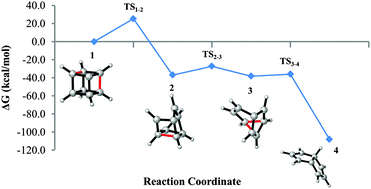
Born–Oppenheimer molecular dynamics simulations and high-level ab initio computations predict that the cage-opening rearrangement of the cubyl cation to the 7H+-pentalenyl cation is feasible in the gas phase. The rate-determining step is the formation of the cuneyl cation with an activation barrier of 25.3 kcal mol−1 at the CCSD(T)/def2-TZVP//MP2/def2-TZVP level. Thus, the cubyl cation is kinetically stable enough to be formed and trapped at moderate temperatures, but it may be rearranged at higher temperatures.

 Please wait while we load your content...
Please wait while we load your content...