Beyond ketonization: selective conversion of carboxylic acids to olefins over balanced Lewis acid–base pairs†
Abstract
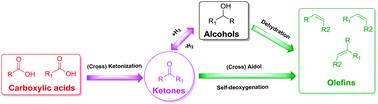
We report the direct conversion of mixed carboxylic acids to C=3–C=6 olefins with up to 60 mol% carbon yield through cascade (cross) ketonization, (cross) aldolization and self-deoxygenation reactions. Co-feeding hydrogen provides an additional ketone hydrogenation/dehydration pathway to a wider range of olefins.

 Please wait while we load your content...
Please wait while we load your content...