Chemo- and regioselective reductive deoxygenation of 1-en-4-yn-ols into 1,4-enynes through FeF3 and TfOH co-catalysis†
Abstract
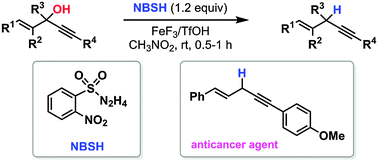
We report chemo- and regioselective direct reductive deoxygenation of 1-en-4-yn-3-ols into 1,4-enynes through FeF3 and TfOH cooperative catalysis under NBSH-mediated conditions. Further, we show the efficient synthesis of a pharmaceutically significant 1,4-enyne. The present methodology can also be used for chemo- and regioselective direct deoxygenation of other alcohols.

 Please wait while we load your content...
Please wait while we load your content...