Unified biogenesis of ambiguine, fischerindole, hapalindole and welwitindolinone: identification of a monogeranylated indolenine as a cryptic common biosynthetic intermediate by an unusual magnesium-dependent aromatic prenyltransferase†
Abstract
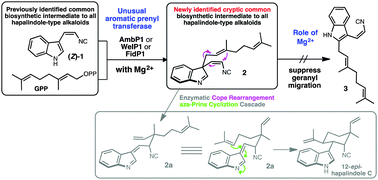
Biochemical characterization of aromatic prenyltransferase AmbP1 and its close homologs WelP1/FidP1 in hapalindole-type alkaloid biosynthetic pathways is reported. These enzymes mediate the magnesium-dependent selective formation of 3-geranyl 3-isocyanovinyl indolenine (2) from cis-indolyl vinyl isonitrile and geranyl pyrophosphate. The role of the magnesium cofactor in AmbP1/WelP1/FidP1 catalysis is unusual for a microbial aromatic prenyltransferase, as it not only facilitates the formation of 2 but also prevents its rearrangement to an isomeric 2-geranyl 3-isocyanovinyl indole (3). The discovery of 2 as a cryptically conserved common biosynthetic intermediate to all hapalindole-type alkaloids suggests an enzyme-mediated Cope rearrangement and aza-Prins-type cyclization cascade is required to transform 2 to a polycyclic hapalindole-like scaffold.

 Please wait while we load your content...
Please wait while we load your content...