An expedient synthesis of functionalized 1,4-diketone-derived compounds via silyloxyallyl cation intermediates†
Abstract
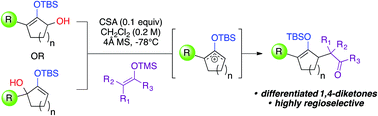
Herein we describe a new method, enabling the synthesis of highly functionalized 1,4-diketones that are readily differentiated as monosilylenol ethers under Brønsted acid catalysis. This synthetically useful chemistry exploited an intermediacy of unsymmetrical silyloxyallyl cations, which were directly captured by silyl enolates to create the targeted α,α carbon–carbon linkages in a regioselective manner. Our reaction conditions proved to be mild, rendering the silylenol ether functionalities intact.

 Please wait while we load your content...
Please wait while we load your content...