Backbone assignment for minimal protein amounts of low structural homogeneity in the absence of deuteration†
Abstract
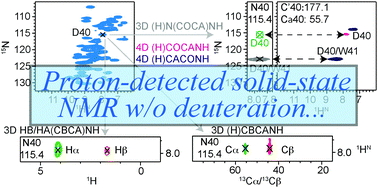
NMR characterization of many proteins is limited by low expression, hurdles for deuteration, and poor sample homogeneity. We introduce a set of high-dimensionality proton-detected experiments developed for unambiguous resonance assignments of such proteins, which we could successfully apply to a 1 mg amount of non-deuterated Tau paired helical filaments.

 Please wait while we load your content...
Please wait while we load your content...