A quinoidal bis-phenalenyl-fused porphyrin with supramolecular organization and broad near-infrared absorption†
Abstract
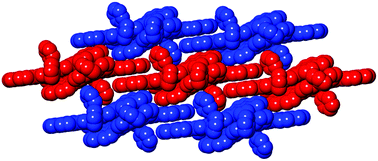
A bis-phenalenyl-fused porphyrin has been synthesized by thermal dehydro-aromatization reaction regioselectively as a single syn-isomer. X-ray crystal structure revealed that both phenalenyl units of this porphyrin have close π–π contacts forming continuous network of interacting porphyrin rings. A broad and intense NIR absorption can be attributed to quinoidal character of bis-phenalenyl-fused porphyrin.

 Please wait while we load your content...
Please wait while we load your content...