Transition-metal free reactions of boronic acids: cascade addition – ring-opening of furans towards functionalized γ-ketoaldehydes†
Abstract
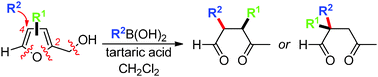
We describe the first ring-opening of furfuryl alcohols with boronic acids to afford functionalized γ-ketoaldehydes. The transformation builds a new C–C bond at the original C-4 of the starting furan, and tolerates ring-substitution at C-3 and C-4 positions. The reaction takes place under metal-free conditions by promotion with tartaric acid.

 Please wait while we load your content...
Please wait while we load your content...