A new type of oxidative addition of an iodoarene to a Pd(ii) complex†
Abstract
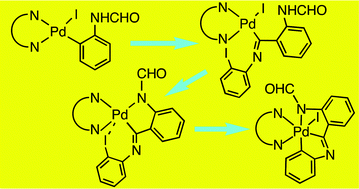
Oxidative addition of N-(2-iodophenyl)formamide to Pd(dba)2 (dba = dibenzylideneacetone) in the presence of 4,4′-ditertbutyl-2,2′-bipyridine (tBubpy) produces [Pd(C6H4NHCHO-2)I(tBubpy)] (1) which inserts 2-iodophenyl isocyanide to give [Pd{C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H4I-2)C6H4NHCHO-2}I(tBubpy)] (2). Dehydroiodination of 2 with Tl(acac) (acacH = acetylacetone) gives the stable Pd(IV) complex OC-6-35-[Pd{C,N,N–C(
NC6H4I-2)C6H4NHCHO-2}I(tBubpy)] (2). Dehydroiodination of 2 with Tl(acac) (acacH = acetylacetone) gives the stable Pd(IV) complex OC-6-35-[Pd{C,N,N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H4-2)C6H4NCHO-2}I(tBubpy)] (4) likely resulting from the spontaneous oxidative addition of the I–Ar moiety present in the unstable intermediate Pd(II) complex [Pd{C,N–C(
NC6H4-2)C6H4NCHO-2}I(tBubpy)] (4) likely resulting from the spontaneous oxidative addition of the I–Ar moiety present in the unstable intermediate Pd(II) complex [Pd{C,N–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H4I-2)C6H4NCHO-2}(tBubpy)] (3). The crystal structure of 4 shows various C–H⋯O hydrogen bonds resulting in chains of dimers stacked along the a axis.
NC6H4I-2)C6H4NCHO-2}(tBubpy)] (3). The crystal structure of 4 shows various C–H⋯O hydrogen bonds resulting in chains of dimers stacked along the a axis.

 Please wait while we load your content...
Please wait while we load your content...