Synthesis of di-, tri- and tetracyclopropylhydrazines†
Abstract
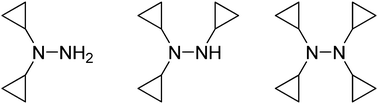
Previously unknown 1,1-dicyclopropylhydrazine was obtained in two steps starting from dicyclopropylamine. It serves as a convenient starting material to tri- and tetracyclopropylhydrazines, which have not been described in the literature either. Tricyclopropylhydrazine was prepared in an overall four-step sequence featuring the de Meijere–Chaplinski modification of the Kulinkovich reaction as a key step. Tetracyclopropylhydrazine was obtained by the reductive amination of the cyclopropanone ethyl trimethylsilyl acetal with 1,1-dicyclopropylhydrazine or with the parent hydrazine. Synthetic utility of these cyclopropylhydrazine building blocks is presented as well.

 Please wait while we load your content...
Please wait while we load your content...