Racemic drug resolution: a comprehensive guide
Abstract
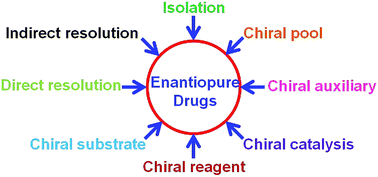
The human body is amazingly chirally selective, and consequently enantiopure drugs are essential for treating disease. Nearly 50% of drugs are chiral wherein pharmacological activity resides with the eutomer (pharmacologically active enantiomer) whereas the distomer (inactive/less potent/equally potent/different pharmacological activity/toxic enantiomer) metabolizes by a different pathway and creates unnecessary burden on the body. A teratogenic (toxic) isomer in a racemic drug creates side-effects, genetic diseases, or may cause death in the case of high dosage. Nowadays, market approval of enantiopure drugs has increased exponentially whereas it has tremendously decreased for racemic and achiral drugs. An expensive process is the main drawback in obtaining enantiopure drugs. Different methods such as chromatography, spectroscopy, and thermal analysis are available for optical purity determination. However, only a few chromatographic methods are useful on an industrial scale. In the present review, racemic resolution and optical purity determination methods are discussed with examples. In addition to this, eutomers and distomers of different drugs containing a single chiral center are also reported.

 Please wait while we load your content...
Please wait while we load your content...