Multivariate optimization, preconcentration and determination of mercury ions with (1-(p-acetyl phenyl)-3-(o-methyl benzoate)) triazene in aqueous samples using ICP-AES†
Abstract
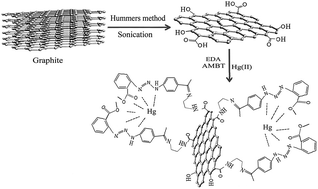
A new modified graphene oxide (GO) was prepared as a solid adsorbent and used for the preconcentration of trace amounts of Hg(II) ions in water samples prior to the determination by inductively coupled plasma atomic emission spectrometry. The method was based on the sorption of analyte on GO which is functionalized by a new ligand: (1-(p-acetyl phenyl)-3-(o-methyl benzoate) triazene (AMBT)). In the adsorption step, central composite design was used to evaluate and determine the optimum level of considered variables (pH, amount of adsorbent and adsorption time). In the desorption step, some factors such as the type, concentration, amount and flow rate of eluting agent were investigated. Many common ions did not interfere. Under the optimal conditions, the adsorption capacity, limit of detection and relative standard deviation were found to be 80 mg g−1, 0.1 μg L−1 and 2.5% (n = 3) respectively. The present method was successfully applied for the determination of mercury ions in real matrix samples.

 Please wait while we load your content...
Please wait while we load your content...