A kinetic spectrofluorometric method, aided by chemometrics, for the analysis of sibutramine, indapamide and hydrochlorothiazide compounds found in weight-reducing tonic samples
Abstract
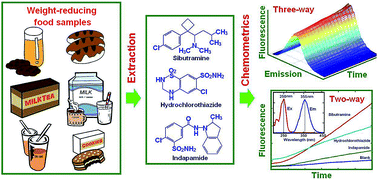
An uncomplicated and sensitive kinetic spectrofluorometric method was developed for the simultaneous analysis of sibutramine, indapamide, and hydrochlorothiazide commonly found in weight-reducing health foods. The mentioned compounds were oxidized by cerium(IV) ammonium sulfate dissolved in an acidic solution. The kinetic-fluorescence spectrum of the produced cerium(III) was recorded between 0 and 600 s reaction time at the excitation wavelength of 250 nm. It was found that the fluorescence intensity was proportional to the concentration of the compounds, and their linear ranges were 0.005–0.12, 0.03–1.44 and 0.01–0.20 μg mL−1; the corresponding detection limits were 1.6, 8.3 and 6.0 ng mL−1 for sibutramine, indapamide and hydrochlorothiazide, respectively. The measured three- and two-way data of the mixtures were processed by chemometrics (included methods: parallel factor analysis (PARAFAC), partial least squares (PLS), principal component regression (PCR), and radial basis function-artificial neural network (RBF-ANN)). The proposed method was applied for the simultaneous determination of the analytes in weight-reducing food samples, and the results were comparable with those from the reference HPLC method.

 Please wait while we load your content...
Please wait while we load your content...