Comparison of the specificity and affinity of surface immobilised Affimer binders using the quartz crystal microbalance†
Abstract
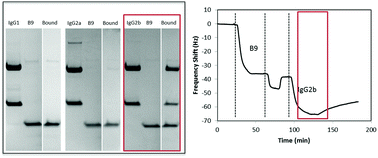
To enable multiplexed protein analysis through the use of microarrays, reliable molecules capable of specifically binding to a protein of interest with high affinity are required. Further, this specificity and affinity must be retained upon immobilization to the microarray surface. This study investigates the performance of surface bound Affimer proteins, comparing the affinity and specificity of different binders for closely related immunoglobulin molecules using the quartz crystal microbalance with dissipation monitoring (QCM-D). It is demonstrated that the surface bound Affimer proteins are highly specific, differentiating between their target IgG and other closely related IgG subclasses. The binding affinities of the protein aptamers for their target IgG molecules are determined to be in the nanomolar range, comparable to typical antibody–antigen binding affinities. While measurements herein are done using QCM-D, the high specificity and binding affinities of the surface bound Affimer proteins opens applications in a range of microarray biosensors.

 Please wait while we load your content...
Please wait while we load your content...