A ratiometric solvent polarity sensing Schiff base molecule for estimating the interfacial polarity of versatile amphiphilic self-assemblies†
Abstract
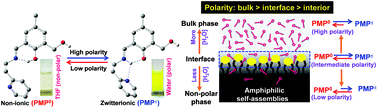
A newly synthesised Schiff base molecule (PMP) existing in equilibrium between non-ionic and zwitterionic forms displays solvent polarity induced ratiometric interconversion from one form to another, such novelty being useful to detect the medium polarity. The specific interface localisation of PMP in versatile amphiphilic self-assembled systems has been exploited to monitor their interfacial polarity by evaluating such interconversion equilibrium with simple UV-Vis spectroscopy. In spite of the large differences in pH and/or viscosity between the bulk and interface, the unchanged equilibrium between the two molecular forms on varying the medium pH or viscosity provides a huge advantage for the exclusive detection of interfacial polarity.


 Please wait while we load your content...
Please wait while we load your content...