On-line chiral analysis using the kinetic method†
Abstract
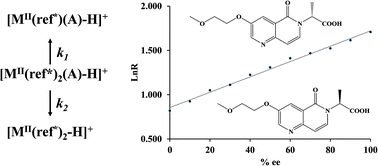
Chiral analysis of constituents in solution-phase reaction mixtures can be performed by tandem mass spectrometry using the kinetic method to determine the enantiomeric excess (ee). Simply diluting an aliquot of a reaction mixture, adjusting the pH, and adding reagents necessary to form a chiral cluster ion allows chiral analysis. The product of a stereospecific N-selective alkylation reaction, 2-(3-(2-methoxyethoxy)-5-oxo-1,6-naphthyridin-6(5H)-yl)propanoic acid, was monitored for ee during the course of reaction, and it showed the expected inversion without ee erosion. Base-catalyzed racemization of the reaction product showed the expected decrease in ee as the reaction proceeded. The base-catalyzed racemization of ibuprofen was monitored on-line, providing near real-time data on ee.

 Please wait while we load your content...
Please wait while we load your content...