A method developed to fractionate intact proteins based on capillary electrophoresis
Abstract
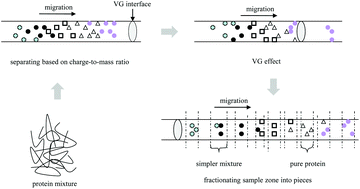
Reduction in the sample complexity enables more thorough intact protein analysis using MS-based proteomics. A capillary electrophoresis method, namely the velocity gap mode of capillary electrophoresis (VGCE), is proposed to separate protein mixtures with high resolution. Although the separation mechanism of VGCE is also based on the difference of the mass-to-charge ratios of the proteins, it fractionates the sample zone into small pieces of subunits. In this way, the resolution can be dramatically improved due to less longitudinal dispersion of the sample. The effect of the new approach is evaluated by separation of three groups of reference protein mixtures, i.e. a mixture of lysozyme and BSA; a mixture of lysozyme, β-lactoglobulin, and ribonuclease A; and a mixture of cytochrome C, lysozyme, BSA, β-lactoglobulin, ribonuclease A, conalbumin, carbonic anhydrase, and hemoglobin. The results indicate that the new approach shows great potential to couple with MS for top-down analysis of complex mixtures.
 Please wait while we load your content...
Please wait while we load your content...