Enantioselective, convergent synthesis of the ineleganolide core by a tandem annulation cascade†
Abstract
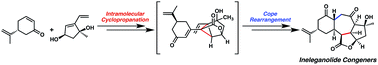
An enantioselective and diastereoselective approach toward the synthesis of the polycyclic norditerpenoid ineleganolide is disclosed. A palladium-catalyzed enantioselective allylic alkylation is employed to stereoselectively construct the requisite chiral tertiary ether and facilitate the synthesis of a 1,3-cis-cyclopentenediol building block. Careful substrate design enabled the convergent assembly of the ineleganolide [6,7,5,5]-tetracyclic scaffold by a diastereoselective cyclopropanation–Cope rearrangement cascade under unusually mild conditions. Computational evaluation of ground state energies of late-stage synthetic intermediates was used to guide synthetic development and aid in the investigation of the conformational rigidity of these highly constrained and compact polycyclic structures. This work represents the first successful synthesis of the core structure of any member of the furanobutenolide-derived polycyclic norcembranoid diterpene family of natural products. Advanced synthetic manipulations generated a series of natural product-like compounds that were shown to possess selective secretory antagonism of either interleukin-5 or interleukin-17. This bioactivity stands in contrast to the known antileukemic activity of ineleganolide and suggests the norcembranoid natural product core may serve as a useful scaffold for the development of diverse therapeutics.


 Please wait while we load your content...
Please wait while we load your content...