Iron–nickel hexacyanoferrate bilayer as an advanced electrocatalyst for H2O2 reduction†
Abstract
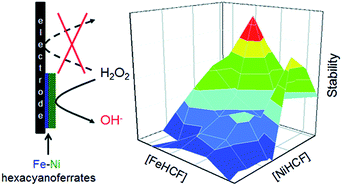
An advanced electrocatalyst for H2O2 reduction was synthesized via the deposition of an iron- and nickel hexacyanoferrate bilayer and displayed a similar electrocatalytic activity (≈0.01 cm s−1) and an almost 100-fold improved operational stability compared to Prussian Blue. The electrocatalyst was highly reproducible, with a deviation in both the operational stability and catalytic activity among different modified electrodes of <10%.

 Please wait while we load your content...
Please wait while we load your content...