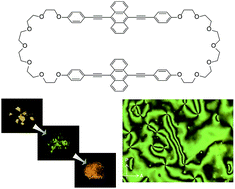
Tuning the thermo- and mechanoresponsive behavior of luminescent cyclophanes†
Abstract
Many cyclophanes have been investigated in dilute solution, where their internal cavities are accessible for supramolecular interactions. However, their photophysical properties in the solid state remain largely unexplored. We here report a new mechano- and thermoresponsive luminescent cyclophane that is comprised of two 9,10-bis(phenylethynyl)anthracene moieties and features two hexaethylene glycol bridges. The compound was found to exhibit a nematic liquid-crystalline phase at elevated temperature. X-ray diffraction patterns confirm that thermal and mechanical treatments induce changes in the molecular assembly, which are the basis for the observed photoluminescent color variations. The stimuli-responsive behavior of the new compound is quite different from that of a previously reported cyclophane with similar structure but shorter bridges. Thus, merely changing the ring size is an effective tool to tailor the stimuli-responsiveness and the phase behaviour of luminescent cyclophanes.

 Please wait while we load your content...
Please wait while we load your content...