Utilising alternative modifications of α-olefin end groups to synthesise amphiphilic block copolymers†
Abstract
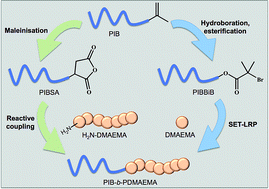
Amphiphilic block copolymers comprised of polyisobutylene (PIB) and poly(2-(dimethylamino)ethyl methacrylate) (DMAEMA) have been synthesised. This has been achieved via two new synthetic processes. Firstly, a PIB macroinitiator was synthesised from PIB that is primarily terminated by an α-olefin, hydroboration/oxidation of the terminal α-olefin followed by esterification with α-bromoisobutyryl bromide furnished the desired macroinitiator. The PIB macroinitiator was then utilised to polymerise DMAEMA by SET-LRP at different block lengths of PDMAEMA. However, end fidelity of the PIB macroinitiator was low (60%) and as such synthesising a block of PDMAEMA with a predictable degree of polymerisation was not possible. PIB-b-PDMAEMA block copolymers were also synthesised by reactive coupling of functional homopolymers, primary amine terminated PDMAEMA and polyisobutenyl succinic anhydride (PIBSA). Variable molecular weight well-defined PDMAEMA was synthesised by SET-LRP using an azide bearing initiator followed by transformation of the azide end group to a primary amine. All primary amine functionalised PDMAEMA was then coupled with PIBSA to synthesise PIB-b-PDMAEMA, demonstrated by GPC. However, the capabilities of this synthetic approach was limited as more than one PDMAEMA may couple to the PIBSA, therefore not synthesising pure PIB-b-PDMAEMA copolymers.

 Please wait while we load your content...
Please wait while we load your content...