Development of an AIE based fluorescent probe for the detection of nitrate anions in aqueous solution over a wide pH range†
Abstract
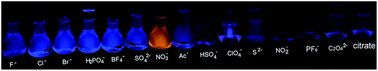
A new type of water-soluble anion fluorescent probe based on ionic interaction-triggered AIE is reported. The positively-charged probe displayed highly selective sensing and imaging of NO3− anions over other environmentally and biologically relevant species through a significant AIE turn-on fluorescence signal in aqueous solution over a wide pH range. The hydrophobic aggregation tendency of the probe itself in aqueous solution was supposed to be beneficial for the target anion to overcome the high dielectric constant of water. As a result, ionic interaction between the positively-charged probe and the negative NO3− anion occurred in aqueous media and thereby an AIE-based selective turn-on fluorescent signal was achieved. These results demonstrate that an ionic interaction triggered AIE can be designed as a new sensing mechanism for the detection of anions in aqueous media and living cells.

 Please wait while we load your content...
Please wait while we load your content...