C–H⋯O (ether) hydrogen bonding along the (110) direction in polyglycolic acid studied by infrared spectroscopy, wide-angle X-ray diffraction, quantum chemical calculations and natural bond orbital calculations
Abstract
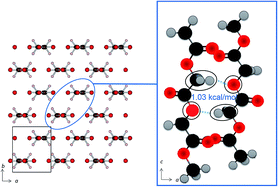
Polyglycolic acid (PGA), a biodegradable polyester with a simple molecular structure, shows an abnormally high melting point of 220 °C which is the highest among biopolyesters. In order to investigate this peculiar physical property and the structure of PGA, its temperature-dependent infrared (IR) spectra and wide angle X-ray diffraction (WAXD) were measured. Of note in the IR spectra is that among the many bands, those due to antisymmetric and symmetric CH2 stretching modes and that due to the C–O–C stretching mode show a significantly lower wavenumber shift with temperature increase. These experimental results suggest the existence of weak C–H⋯O (ether) hydrogen bonding along the (110) direction in PGA. WAXD results support this suggestion by an observed noticeable decrease of a unit cell length along the (110) direction. The natural bond orbital (NBO) calculations indicate there is weak intermolecular bonding between the C–H bond of the CH2 group and the oxygen atom of the –C–O–C– linkage. The quantum chemical calculations of the IR spectra were carried out for models of PGA based on the crystalline structure by using a partial optimization in the normal mode. The results can qualitatively explain the observed temperature dependence of the CH2 stretching modes of PGA, which is due to weakening of the C–H⋯O interactions among polymer chains with a temperature increase. All the experimental and theoretical calculation results suggest that there is weak hydrogen bonding between the CH2 group and the ether oxygen atom. This intermolecular interaction may be the cause for the abnormally high melting point of PGA.

 Please wait while we load your content...
Please wait while we load your content...