Thermodynamically driven, syn-selective vinylogous aldol reaction of tetronamides†
Abstract
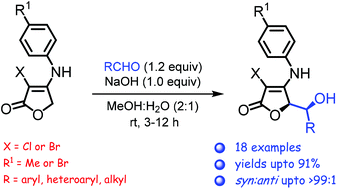
A stereoselective vinylogous aldol reaction of N-monosubstituted tetronamides with aldehydes is described. The procedure is simple and scalable, works well with both aromatic and aliphatic aldehydes, and affords mainly the corresponding syn-aldol adducts. In many cases, the latter are obtained essentially free of their anti-isomers (dr > 99 : 1) in high yields (70–90%). Experimental and computational studies suggest that the observed diastereoselectivity arises through anti–syn isomer interconversion, enabled by an iterative retro-aldol/aldol reaction.
 Please wait while we load your content...
Please wait while we load your content...