New enolate-carbodiimide rearrangement in the concise synthesis of 6-amino-2,3-dihydro-4-pyridinones from homoallylamines†
Abstract
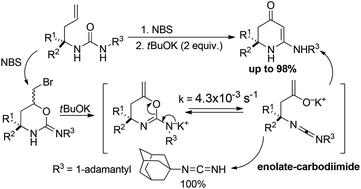
Three-step synthesis of 6-amino-2,3-dihydro-4-pyridinones from homoallylamines involving NBS-mediated cyclization of N-(3-butenyl)ureas to 6-(bromomethyl)-2-iminourethanes, dehydrohalogenation and a novel rearrangement as a key step has been developed. The scope and limitations of the method, as well as the mechanism of the rearrangement, supported by kinetic studies and the isolation of N-(1-adamantyl)carbodiimide, are discussed. The final products, imino-analogues of well known piperidine-2,4-diones, are promising building blocks in the synthesis of bio-/pharmacological compounds.
 Please wait while we load your content...
Please wait while we load your content...