Methionine sulfoxide profiling of milk proteins to assess the influence of lipids on protein oxidation in milk
Abstract
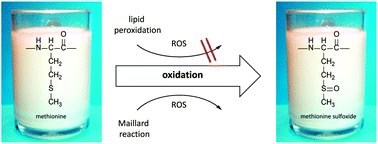
Thermal treatment of milk and milk products leads to protein oxidation, mainly the formation of methionine sulfoxide. Reactive oxygen species, responsible for the oxidation, can be generated by Maillard reaction, autoxidation of sugars, or lipid peroxidation. The present study investigated the influence of milk fat on methionine oxidation in milk. For this purpose, quantitative methionine sulfoxide profiling of all ten methionine residues of β-lactoglobulin, α-lactalbumin, and αs1-casein was carried out by ultrahigh-performance liquid chromatography–electrospray ionization tandem mass spectrometry with scheduled multiple reaction monitoring (UHPLC–ESI–MS/MS–sMRM). Analysis of defatted and regular raw milk samples after heating for up to 8 min at 120 °C and analysis of ultrahigh-temperature milk samples with 0.1%, 1.5%, and 3.5% fat revealed that methionine oxidation of the five residues of the whey proteins and of residues M 123, M 135, and M 196 of αs1-casein was not affected or even suppressed in the presence of milk fat. Only the oxidation of residues M 54 and M 60 of αs1-casein was promoted by lipids. In evaporated milk samples, formation of methionine sulfoxide was hardly influenced by the fat content of the samples. Thus, it can be concluded that lipid oxidation products are not the major cause of methionine oxidation in milk.
- This article is part of the themed collection: The Maillard reaction in food and nutrition

 Please wait while we load your content...
Please wait while we load your content...