A series of fluorinated phenylpyridine-based electron-transporters for blue phosphorescent OLEDs†
Abstract
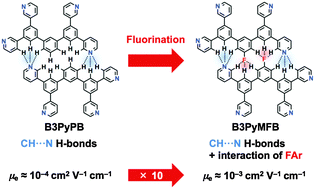
A series of fluorinated phenylpyridine-based electron-transport materials (ETMs) for organic light-emitting devices (OLEDs) based on 3,5,3′′,5′′-tetra-3-pyridyl-[1,1′;3′,1′′]terphenyl (B3PyPB) were developed, and the influence of fluorine atom(s) in the core skeleton on their physical properties, such as molecular orientation, electron-transport and electron-injection, was investigated. The monofluorinated ETM exhibited a relatively large orientation order parameter of −0.23 and an electron mobility of 10−3 cm2 V−1 s−1, which was 10 times higher than that of its nonfluorinated counterpart because of concerted intermolecular interactions involving not only the CH⋯N hydrogen bonds but also the aromatic fluorine atoms, in addition to π–π stacking. Surprisingly, the difluorinated ETM exhibited a smaller molecular orientation order parameter than the mono- and nonfluorinated ETMs, likely because of the poor intermolecular interactions between the fluorine atoms and nitrogen lone pairs. Blue phosphorescent OLEDs fabricated using the monofluorinated ETM had a low operation voltage of 3.5 V at 1000 cd m−2.

 Please wait while we load your content...
Please wait while we load your content...