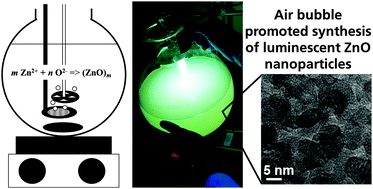
Air bubble promoted large scale synthesis of luminescent ZnO nanoparticles
Abstract
Luminescent zinc oxide nanoparticles were synthesized via a facile one-pot sol–gel synthesis route using zinc acetate dihydrate and potassium hydroxide as cheap precursors. It is demonstrated that dissolved oxygen plays a key role in the synthesis of nanoparticles. For larger scale synthesis batches, it is therefore crucial to provide sufficient oxygen supply during reaction, as otherwise, the formation of ZnO nanoparticles does not occur. A concept is presented to achieve a large gas to liquid interface by introducing air bubbles into the reaction system to ultimately promote ZnO nanoparticle formation at larger scale batches. The flow rate of air during synthesis directly influences the yield and the luminescence intensity of the nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...