The effect of regioisomerism on the crystal packing and device performance of desymmetrized anthradithiophenes†
Abstract
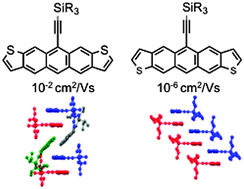
Anthradithiophenes (ADTs) are typically synthesized as inseparable mixtures of regioisomers. In this paper, we describe the synthesis of desymmetrized anthradithiophenes containing one trialkylsilylethyne solubilizing group, which allowed chromatographic separation of the three resulting isomers. Cyclic voltammograms, as well as absorption and emission spectra for all isomers, were nearly identical. However, X-ray crystallography revealed that the positions of the sulfur atoms in each isomer strongly influence crystal packing, corroborating calculations that show the S–π interaction to be less stabilizing than the C–H–π interaction. Isomer 3c packs in a pseudo 1-D fashion while isomers 3a and 3b pack as isolated π-stacked pairs. Isomer 3c shows a field-effect mobility four orders of magnitude higher than isomers 3a and 3b, presumably due to this difference in packing motif.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry C Hot Papers

 Please wait while we load your content...
Please wait while we load your content...