Pure aromatic hydrocarbons with rigid and bulky substituents as bipolar hosts for blue phosphorescent OLEDs†
Abstract
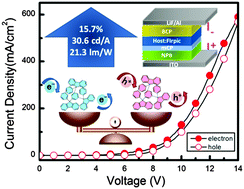
Four pure hydrocarbon molecules of 1,3,5-tris(9-phenyl-9H-fluoren-9-yl)benzene (mTPFB), 4,4′-bis(9-phenyl-9H-fluoren-9-yl)biphenyl (pDPFBP), 1,4-bis(9-phenyl-9H-fluoren-9-yl)benzene (pDPFB) and 1,3-bis(9-phenyl-9H-fluoren-9-yl)benzene (mDPFB), with different connecting modes and cores were prepared in a one-pot reaction. Among them, mTPFB and pDPFDB were newly designed for the study of the structure–property relationship and developing good blue hosts for phosphorescent organic light-emitting devices (PhOLEDs). The four materials show very similar photophysical properties in solution, with almost the same bandgap and triplet energy, as well as similar energy levels. However, their device behavior is quite different with the best performance coming from mTPFB. The conjugation blocked connection mode in the starburst mTPFB molecule with rigid and bulky substituents results in a high triplet energy coupled with good and balanced electron and hole transport, as proved by the internal reorganization energy calculation and mono charge device study, which makes it a good host for a blue phosphor in a PhOLED. The device shows an external quantum efficiency of 15.7% and a current efficiency of 30.6 cd A−1, which is among the best for pure hydrocarbon host based devices.

 Please wait while we load your content...
Please wait while we load your content...