Structural and optical properties of Fe and Zn substituted CuInS2 nanoparticles synthesized by a one-pot facile method
Abstract
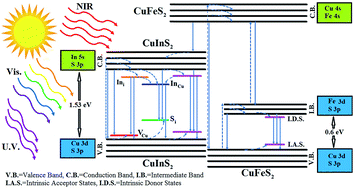
We substitute indium present in the CuInS2 ternary compound by iron and zinc using a facile one-pot synthesis method. The quaternary compound of CuIn1−xMxS2 (M = Fe and Zn) was synthesized by dissolving CuCl, InCl3, FeCl3, Zn(ac)2 and SC(NH2)2 as precursors in 1-octadecene, oleylamine and oleic acid as non-coordinating, coordinating and capping agent solvents, respectively. Oleic acid, oleylamine and thiourea were used respectively as a hard Lewis base, borderline Lewis base (in comparison with oleic acid) and soft Lewis base to form appropriate complexes. The complex formation, structure, and optical properties of CuIn1−xMxS2 (M = Fe and Zn) nanoparticles were studied by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), X-ray photoemission spectroscopy (XPS), transmission electron microscopy (TEM), and ultraviolet-visible-near infrared (UV-vis-NIR) and photoluminescence (PL) spectroscopy. The absorption edge of solid solution samples can be tuned from 745 nm (visible) to 1200 nm (near infrared) by substitution of In3+ by Zn2+ and Fe3+, respectively.

 Please wait while we load your content...
Please wait while we load your content...