Boron ketoiminate-based conjugated polymers with tunable AIE behaviours and their applications for cell imaging†
Abstract
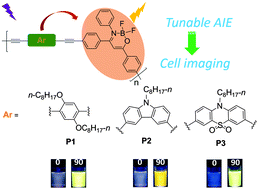
Three new boron ketoiminate-based conjugated polymers P1, P2, and P3 were designed and synthesized through the Sonogashira coupling reaction of 4,6-bis(4-bromophenyl)-2,2-difluoro-3-phenyl-2H-1,3,2-oxazaborinin-3-ium-2-uide (M1) with 1,4-diethynyl-2,5-bis(octyloxy)benzene (M2), 3,6-diethynyl-9-octyl-9H-carbazole (M3) and 3,7-diethynyl-10-octyl-10H-phenothiazine-S,S-dioxide (M4), respectively. All the resulting polymers showed obvious aggregation-induced emission (AIE) behaviours. Interestingly, it was found that a great difference in the electron-donating abilities of the D–A type polymer linkers can lead to the unique AIE behaviour of the alternating polymers in the aggregate state, which provides us with a practical strategy to design tunable AIE-active conjugated polymers. Most importantly, studies on MCF-7 breast cancer cell imaging revealed that the nanoparticles fabricated from the conjugated polymers could serve as promising fluorescent probes with low cytotoxicity and high photostability.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry B Hot Papers

 Please wait while we load your content...
Please wait while we load your content...