Octacalcium phosphate – a metastable mineral phase controls the evolution of scaffold forming proteins†
Abstract
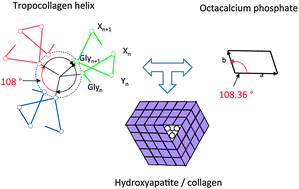
The molecular structure of collagen type 1 can be understood as the result of evolutionary selection in the process of formation of calcium phosphate based biocomposites acting as load bearing components in living organisms. The evolutionary selection fulfills the principle of ‘survival of the fittest’ in a particular biological environment. Disk-like post-nucleation complexes of Ca2(HPO4)32− organized in ribbon-like assemblies in the metastable octacalcium phosphate (OCP) phase, and Ca3 triangles in the stable HAP phase had formed the crystallographic motifs in this selection process. The rotational as well as the translational symmetry of the major tropocollagen (TC) helix agree nearly perfectly with the corresponding symmetries of the OCP structure. The sequence of (Gly–X–Y) motifs of the three α chains constituting the TC molecule enables an optimized structural fit for the nucleation of Ca3 triangles, the directed growth of nanostructured OCP, and the subsequent formation of hydroxyapatite (HAP) in collagen macrofibrils by a topotaxial transition. The known connection between genetic defects of collagen type 1 and Osteogenesis imperfecta should motivate the search for similar dependences of other bone diseases on a disturbed molecular structure of collagen on the genetic scale.

 Please wait while we load your content...
Please wait while we load your content...