Thermoresponsive block copolymer micelles with tunable pyrrolidone-based polymer cores: structure/property correlations and application as drug carriers†
Abstract
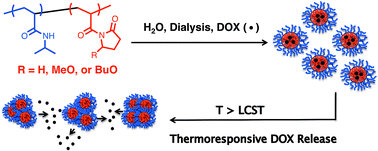
A–B block copolymer micelles comprised of thermoresponsive hydrophilic PNIPAAm (poly(N-isopropylacrylamide)) coronae and hydrophobic PNP (poly(N-acryloyl-2-pyrrolidone)), PMNP (poly(N-acryloyl-5-methoxy-2-pyrrolidone)), or PBNP (poly(N-acryloyl-5-butoxy-2-pyrrolidone)) cores were examined to identify how systematic adjustments to core–segment structure affect micellar physicochemical properties, drug loading efficiency (DLE), and thermoresponsive drug release among these novel systems. Critical micelle concentrations (CMCs) were found to decrease by two orders of magnitude in the order of PNIPAAm–PNP, PNIPAAm–PMNP, and PNIPAAm–PBNP indicating that minor modifications to the pyrrolidone scaffold significantly affect its hydrophobic character. Moreover, the structural modifications were also found to influence micelle size and intermicellar aggregation that occurs above the lower critical solution temperature (LCST). In line with the CMC data, DLE values of doxorubicin-loaded (i.e., DOX-loaded) micelles increase in the order of PNIPAAm–PNP, PNIPAAm–PMNP, and PNIPAAm–PBNP, a trend attributed to enhanced cohesive forces (i.e. London dispersion forces) between DOX and core as the latter becomes more hydrophobic. When heated above the LCST, DOX release decreases in the order of PNIPAAm–PNP, PNIPAAm–PMNP, and PNIPAAm–PBNP suggesting that release processes are impeded by the cohesive forces responsible for efficient encapsulation. Finally, cytotoxicity assays performed above the LCST reveal that DOX-loaded micelles are as cytotoxic as the free drug in formulations where DOX concentrations are equivalent.

 Please wait while we load your content...
Please wait while we load your content...