Photocatalytic reduction of CO2 with water promoted by Ag clusters in Ag/Ga2O3 photocatalysts†
Abstract
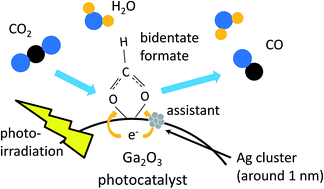
Ag loaded Ga2O3 (Ag/Ga2O3) photocatalysts were prepared by an impregnation method and examined for the photocatalytic reduction of CO2 with water where CO, H2 and O2 were formed as products. TEM and X-ray absorption near edge structure (XANES) measurements revealed that around 1 nm sized Ag clusters were formed predominantly in an active Ag/Ga2O3 sample while partially oxidized large Ag particles with the size of several–several tens of nm were observed in a less active Ag/Ga2O3 sample. Both Ag L3-edge and O K-edge XANES analyses suggested that the small Ag clusters accepted more electrons in the d-orbitals as a result of the strong interaction with the Ga2O3 surface. In situ FT-IR measurements of the Ag/Ga2O3 samples showed CO3 stretching vibration bands assignable to monodentate bicarbonate and bidentate carbonate species chemisorbed on the Ga2O3 surface, and to monodentate carbonate species on the large Ag particles. Among these chemisorbed species, the monodentate bicarbonate and/or the bidentate carbonate species changed to bidentate formate species, as the reaction intermediate, under UV light irradiation. The bidentate formate species was formed not by the plasmonic excitation of the Ag nanoparticles but by the photoexcitation of the Ga2O3 semiconductor, and the formation process would be promoted at the perimeter of the Ag clusters on the Ga2O3 surface by the effective separation of electron–hole pairs.
- This article is part of the themed collection: Artificial Photosynthesis

 Please wait while we load your content...
Please wait while we load your content...